After performing a master thesis in the Biomedical Photonic Imaging Group (BMPI) of the University of Twente, I graduated from Ecole Centrale de Lyon in 2011. I then came back to the university of Twente to fulfill a PhD in the Physics Of Fluids Group, under the supervision of prof. Michel Versluis, on ultrasound contrast agents and graduated in 2015. I am now associate professor in the Physics Of Fluids Group, working on ultrasound, microscale fluid dynamics, bubble physics, and deep learning for ultrasound super-resolution contrast agents.
Expertise
Physics
- Ultrasound
- Bubbles
- Contrast
- Acoustics
- Pressure
- Droplet
Medicine and Dentistry
- In Vitro
- Combination Therapy
Organisations
My research interests include a broad array of microscale phenomena, in particular these that can lead to the generation of contrast for ultrasound and photoacoustics. Such phenomena often involve cavitation, phase change and heat and mass transfer. I also have a keen interest in therapeutic applications of microbubbles for sonoporation, sonoprinting and gene therapy. Studying microscale physics also requires a precise control of the particles properties, and in particular their size. Part of my research activities therefore focuses on production methods and precise control of agents created using microfluidics. High-speed imaging is a critical tool in such studies and allows for the direct visualization of the response of the contrast agents. The recent arrival of ultrafast plane-wave ultrasound technology combined with monodisperse microbubbles opens new possibilities for highly controlled and localized drug delivery and greatly improved diagnostics for personalized medicine.
Publications
2026
2025
2024
Research profiles
For the past 6 years, I am involved in teaching medical acoustics to master students with Prof. Michel Versluis. I have also supervised several bachelor thesis students and 9 master thesis students.
Affiliated study programs
Courses academic year 2025/2026
Courses in the current academic year are added at the moment they are finalised in the Osiris system. Therefore it is possible that the list is not yet complete for the whole academic year.
- 193542070 - Medical Acoustics
- 193599010 - Internship
- 193599039 - Master Thesis: Physics Aspects
- 193599089 - Master Thesis: General Aspects
- 193650999 - Masters Assignment
- 201700185 - Internship
- 201800344 - Master's Assignment: Physics Aspects
- 201800345 - Master's Assignment: General Aspects
- 202000675 - Project Dynamics and Relativity
- 202000716 - Bachelor Assignment
- 202001433 - Bachelor’s Assignment AM-AP
- 202400274 - Signals and Systems
- 202500396 - Project: Imaging Technologies
- 202500397 - Imaging Techniques
Courses academic year 2024/2025
- 193542070 - Medical Acoustics
- 193599010 - Internship
- 193599039 - Master Thesis: Physics Aspects
- 193599089 - Master Thesis: General Aspects
- 193650999 - Masters Assignment
- 201700185 - Internship
- 201800344 - Master's Assignment: Physics Aspects
- 201800345 - Master's Assignment: General Aspects
- 202000716 - Bachelor Assignment
- 202001433 - Bachelor’s Assignment AM-AP
- 202400273 - Project: Imaging Technologies
- 202400274 - Signals and Systems
- 202400275 - Imaging Techniques
The research projects in which I am involved revolve around the idea of using microscale physics in order to generate contrast or induce therapy down to the single cell level. These mechanisms involve in particular phase change, microbubbles resonance and controlled production of complex particles.
Recently, I have focused on the use of these physical mechanisms combined with deep-learning for super-resolution ultrasound imaging.
Current projects
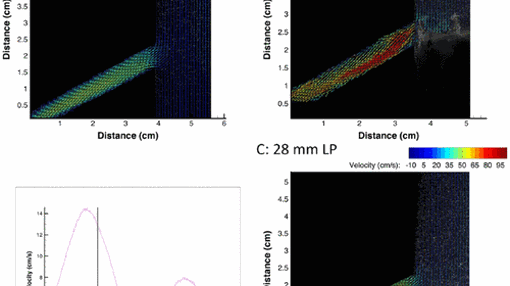
Ultrafast plane-wave ultrasound imaging
Plane-wave ultrasound allows us to image at several thousands of frames per second and thereby image biological processes that are otherwise too fast. In particular, we aim at flow parameters quantification using plane ultrasound and microbubble contrast agents, at a sub-millisecond timescale and resolve the details of the blood flow in relation to stents or vascular diseases.
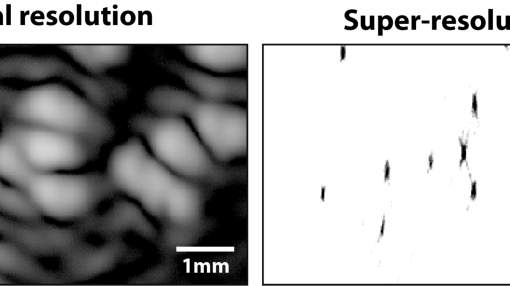
Ultrasound super-resolution from raw ultrasound data using deep learning
Recently, ultrasound localization microscopy (ULM) has received much attention as a method to overcome the diffraction limit in ultrasound imaging. However, ULM relies on low concentrations of microbubbles, ultimately resulting in long acquisition times. In this project, we investigate an alternative super-resolution approach, based on deconvolution of the raw ultrasound data with neural networks. We focus on low-frequency ultrasound in order to image deep in the body, which is needed for cardiovascular diseases or cancer. To that end, we train the neural network to recognize the specific nonlinear feature of echoes generated by monodisperse bubbles (uniform in size).
Lab-on-a-Chip Microfluidics.
The formation of microscopic droplets, bubbles and particles with an accurately controlled and narrow size distribution is crucial in a wide variety of applications and products. For example, in medical applications such as diagnostic ultrasound imaging, targeted drug delivery, and drug inhalation therapy, but also in inkjet printing, cosmetics and in modern food industry.
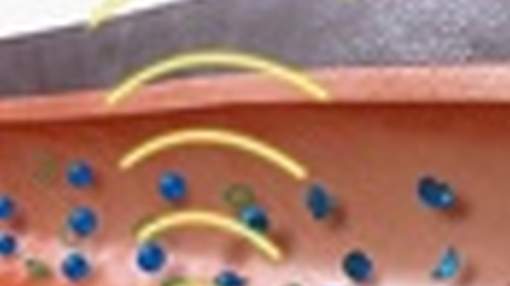
Ultrasound Therapy with microbubbles.
The use of ultrasound contrast agents as local drug delivery systems continues to grow. Microbubbles are an interesting vehicle that can carry a variety of payloads from functional nanoparticles to RNA loaded liposomes. We study here the physical aspects that lead to the controlled release of the payload upon ultrasound exposure. The effects on cells and mechanisms such as sonoporation or sonoprinting, that can temporarily open cell membranes, are also of prime interest. The project includes the study of single bubbles and of their interaction with 3D cell structures, in parallel, and makes use of ultra-high-speed imaging, fluorescence imaging, ultrasound, optical microscopy and confocal imaging.

Ultrasound Imaging.
Contrast agents for ultrasound imaging are used daily in the clinics in order to, for example, assess blood perfusion or give information on tumor malignancy. In this project, we study the formation and the behavior of microbubbles in response to ultrasound in order to find innovative contrast mode for ultrasound imaging, and provide new information for diagnosis.

High-speed imaging.
High-speed imaging is a core technique for the study of ultrasound contrast agents. This project aims at designing and implementing new techniques in order to push further the exploration of small length scales and short time scales.
Finished projects
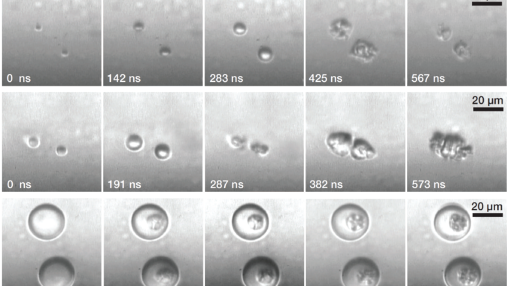
Multimodal agents: ultrasound and photoacoustics
Ultrasound and photoacoustic imaging are complementary by many aspects. Here, we design agents consisting of microbubbles or microcapsules, that are made sensitive to light by the addition of a dye in the bubble oil coating or in the polymer shell of the capsules. The fast heat and mass transfer on the microscale combined with the dynamics of microbubbles allow these agents to mechanically respond to both laser irradiation and ultrasound excitation. These agents could thus be use in a multimodal imaging setting including ultrasound and photoacoustics.
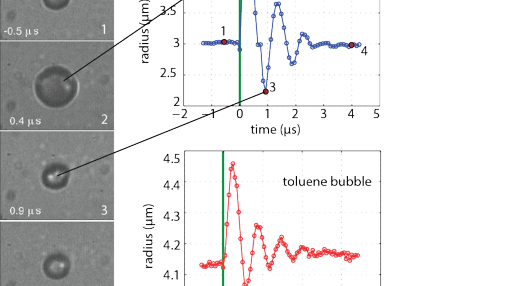
Phase-change agents for ultrasound.
Microbubbles have undeniably proven their value as ultrasound contrast agents, both for imaging and therapy. However, their micron size confines them to the blood vessels, making them exclusively blood pool agents. In this project, we study the vaporization mechanisms of superheated perfluorocarbon droplets that can be made small enough to extravasate and subsequently turned into microbubbles, using ultrasound as trigger. These microbubbles can then be used beyond the endothelial barrier for both diagnosis and therapy.
News on utwente.nl
https://www.utwente.nl/en/tnw/bmpi/news/archive/2014/paper-in-nature-communications/
https://www.utwente.nl/en/news/!/2014/4/315598/novel-contrast-agent-for-medical-imaging
Address

University of Twente
Horst Complex (building no. 20), room ME214A
De Horst 2
7522 LW Enschede
Netherlands
University of Twente
Horst Complex ME214A
P.O. Box 217
7500 AE Enschede
Netherlands